Recently, Hunan Yineng Biopharmaceutical Co., Ltd. (hereinafter referred to as “Yineng Pharmaceutical”) has received the Drug Registration Certificate for Linggui Zhugan Decoction Granules approved and issued by the National Medical Products Administration (NMPA). The product has been approved for marketing as a Class 3.1 new traditional Chinese medicine (TCM). Its approval not only marks a key breakthrough for the enterprise in the field of inheritance and innovation of TCM, but also provides a new therapeutic option that combines traditional efficacy with modern convenience for patients suffering from fluid retention syndrome due to insufficient middle yang.
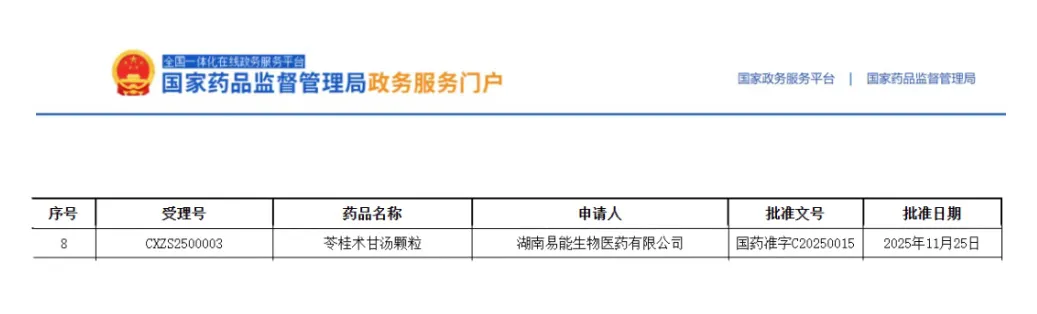
Derived from Synopsis of the Golden Chamber written by Zhang Zhongjing in the Han Dynasty, Linggui Zhugan Decoction Granules is mainly indicated for fluid retention caused by insufficiency of middle yang, with symptoms including stuffiness and fullness in the chest and hypochondria, dizziness, palpitations, shortness of breath accompanied by cough, white and slippery tongue coating, as well as wiry and slippery pulse. In modern clinical practice, it is commonly used to treat chronic bronchitis, bronchial asthma, cardiogenic edema, edema caused by chronic glomerulonephritis, Meniere’s disease, neurosis and other conditions attributed to fluid retention in the middle energizer. Clinically, it is applicable to departments such as respiratory medicine, cardiology and nephrology.
This formula targets a large potential population of users, with clear and sustained clinical demand; its modern granule dosage form is also highly compatible with current medication habits, boasting favorable market acceptance and promotion potential.
Relying on strong R&D capabilities, Yineng Pharmaceutical has systematically carried out research on key technologies such as medicinal material origin control, extraction process optimization, volatile oil extraction and formulation development on the basis of inheriting the essence of ancient prescriptions. It has established a full-process quality control system covering raw materials to finished products, so as to ensure the stable efficacy and controllable quality of the product.
The approval of Linggui Zhugan Decoction Granules is an important achievement of Yineng Pharmaceutical in practicing its corporate development philosophy of “serving the country and safeguarding people’s health”, as well as a rewarding outcome of the team’s R&D investment. Going forward, Yineng Pharmaceutical will accelerate the industrialization and market layout of Linggui Zhugan Decoction Granules, and continue to deepen the R&D of classic TCM prescriptions and new TCM drugs. By virtue of solid scientific research capabilities and technical accumulation, the company will promote the launch of more new TCM products with definite efficacy and broad market prospects, contributing to the high-quality development of the TCM industry.
Post time: Jan-13-2026
